To begin to understand how a cathode-ray TV set works, I could remove one component called the “transistor”, and the picture disappears. It would be an incorrect conclusion to say that the purpose of that transistor is to produce the picture. However, I could argue correctly that the transistor was somehow part of the system required to produce the picture.
If I showed the transistor was particularly “hot” while the TV set was on, producing a picture, it might be reasonable for me to conclude the transistor was involved in producing the picture.
This is the sort of basic approach still used to work out what is going on in the brains and minds of people with Alzheimer’s disease, typical presentations of which might be memory problems. You can see whether removing parts of the brain in humans produces similar effects to the problems in thinking found in Alzheimer’s disease. Or alternatively, you could just try to look at the system of components in the brain which might be contributing to memory in brains working normally.
Whatever, it’s a puzzle. In this particular case, it’s a puzzle to solve correctly.
An innovation culture in the diagnosis of Alzheimer’s disease
David Cameron praised Cambridge Cognition’s work in developing new innovative tests for Alzheimer’s disease in the G8 summit held towards the end of last year.
There has been concern that some individuals with Alzheimer’s disease do not receive their diagnoses in a particularly fast way. A number of explanations for this have been offered, including medical personnel not being able to spot the symptoms of Alzheimer’s disease easily.
It is also helpful to understand what an “innovation” is. An innovation might be a product which enables you do something much more easily, and depends for its success popular uptake by the user. Strictly speaking, paper was an innovation too. However, the rise in cost of diagnosing Alzheimer’s disease, arguably, is an intriguing example of “Baumol’s cost disease“.
Individuals with Alzheimer’s disease have memory problems which are typically not thought to be qualitatively similar to those found in ageing elderly individuals. Often such people have real problems in navigating around environments. It is clearly a very laudable aim to have a bedside test which might be able to alert a physician to an underlying memory problem in Alzheimer’s disease.
The benefits and concerns, and my passing involvement
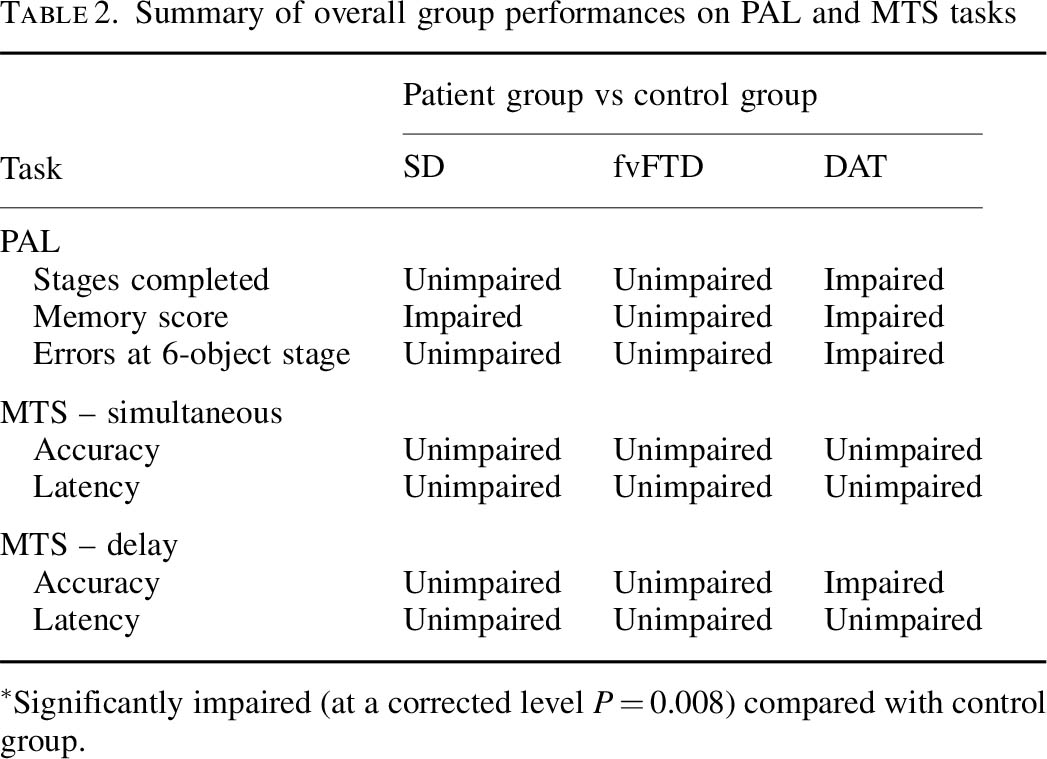
There are a number of important caveats here. Not all dementias are Alzheimer’s disease. There are in fact hundreds of dementias, some of which are reversible. Whatever test is used, the test should be sensitive enough to identify reliably a genuine thinking problem in Alzheimer’s disease, but should not be so ‘broad brush’ the test also misattributes memory problems, say found in the ‘mild cognitive impairment’ or even depression, to Alzheimer’s disease. Such mislabelling can perceivably cause distress, and cause people to be caught up in the medical system for further lengthy tests when they should not have been in the first place. On the other hand, it is of concern that the diagnosis might be missed in some people, and hence the drive from the Department of Health and the Alzheimer’s Society in “The Prime Minister’s Dementia Challenge”.
I wish Cambridge Cognition well, not least because I have worked with CANTAB whilst a graduate student at the University of Cambridge. In fact, some of my papers are cited in their bibliography. Their search facility is here.
The CANTABmobile “paired associates learning” test
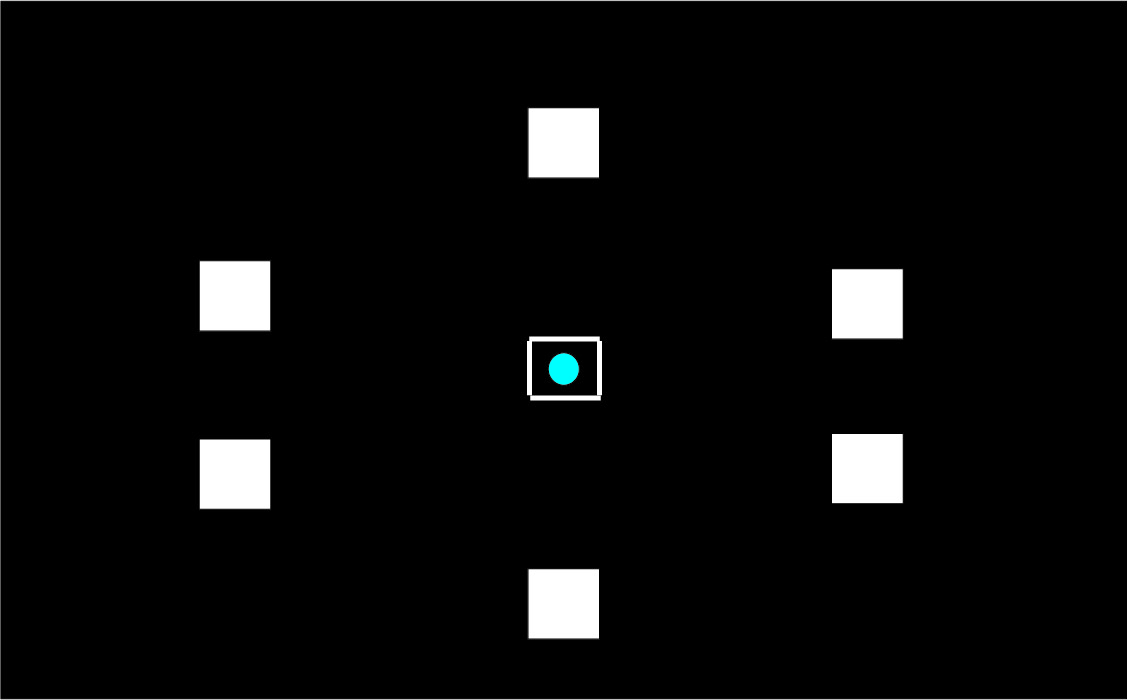
To explain the “paired associates learning” test from first principles, and I’m not using actual screenshots, imagine me presenting you with a number of blank boxes dotted around the screen.
And I open each box in turn and reveal a shape to you. I can present the problem with a varying number of shapes.
After showing you all the shapes, I then present to you a shape and ask you to identify the box in which it was first presented.
Cambridge Cognition in welcoming the Draft National Plan to Address Alzheimer’s disease in my opinion set out entirely correctly the advantages of this computerised testing battery; including fast, not culturally biased, not heavily loading on language, norm-referenced, culturally unbiased, and easy-to-use.
The reasoning behind it being sensitive to early Alzheimer’s disease – but what about mild cognitive impairment?
To understand why the narrative for the test being so attractive in early Alzheimer’s disease, you have to understand that this test has been found to be sensitive to functions of particular brain areas. If you chop out bits of the brain near the front of the head (frontal cortex) or near the ear (temporal cortex), performance on this task is impaired, as Prof Adrian Owen showed when he was a post-doctoral fellow (paper here). With hindsight, perhaps Owen should have looked at the effects of other brain areas further back in the brain, such as the parietal cortex, which are also now thought to be important in memory for spatial cues.
A consistent finding has been loss of brain cells in the “entorhinal cortex”, in the temporal cortex, early in Alzheimer’s disease (see for example here). Therefore, that the paired associates learning test should identify memory problems in early Alzheimer’s disease immediately makes intuitive sense.
But the issues I feel are much more complicated, and I wish Cambridge Cognition well in clarifying them.
If it’s not Alzheimer’s disease, what else could be causing the memory problems?
One possibility is “mild cognitive impairment”. It is described, for example on the authoritative Mayo Clinic website, that:
“Mild cognitive impairment (MCI) is an intermediate stage between the expected cognitive decline of normal aging and the more serious decline of dementia. It can involve problems with memory, language, thinking and judgment that are greater than normal age-related changes. If you have mild cognitive impairment, you may be aware that your memory or mental function has “slipped.””
David Hart, Senior Business Development Manager of Cambridge Cognition, kindly sent Dr Peter Gordon the rationale for the use of the CANTAB task by Dr Andrew Blackwell, their Chief Scientific Officer (as produced on Peter’s blog here).
Cambridge Cognition concede that distinguishing between MCI and Alzheimer’s Disease “is difficult”, but this is a distinction that must be arrived at otherwise a test potentially will give “false positives” – but no test is perfection, and it basically is impossible to strive for perfection. What we all trying avoid is where a test for possible dementia itself is expensive followed by a further expensive investigation to show the original result was a false positive – or as the Express euphemistically called it recently, “Dementia diagnosis proved wrong by new super scanner”. (It is important to state clearly here that no details are given how a diagnosis had been arrived at previously for Ros Davies.)
To give them credit, Cambridge Cognition cite the Chandler et al. (2008) paper, but the full citation of this is “Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association Volume 4, Issue 4, Supplement , Pages T551-T552, July 2008″ – i.e. it is a supplement of abstracts not full papers. This particular abstract can be viewed here.
It is hoped that this full study will have been published elsewhere, and if so Cambridge Cognition will need to update their website with the full paper. Notwithstanding this, the numbers of individuals in each group are disappointingly low: there are seventeen with putative MCI and twelve with putative Alzheimer’s disease.
Is this task actually sensitive and specific?
However, the discussion by Dr Andrew Blackwell and colleagues in his 2004 paper is useful. I have more than a passing interest in that paper as the main author on that paper was one of my PhD supervisors at Cambridge, Prof John Hodges. John has also kindly written one of my three Forewords for my book, “Living well with dementia” to be published on January 14th 2014.
Blackwell remarks correctly that this task has been used to distinguish between unipolar depression and Alzheimer’s disease in Rachel Swainson’s study. But is this enough? I looked to the previous Beats study in “geriatric depressive”, and there was nothing forthcoming there. How confident can one be that only early patients with Alzheimer’s disease, and not those severely depressed or with an underactive thyroid, will perform abnormally on the PAL? Personally, I’m not at all confident yet, despite the Swainson study, but these fears can easily be allayed with a sensitivity/specificity study of much higher power.
Blackwell is however correct in citing my study with Dr Andy Lee in that patients with semantic dementia and behavioural variant frontotemporal dementia are relatively unimpaired, though the clinical presentations of the frontotemporal dementias can be quite clearly different in the clinic from Alzheimer’s disease. Completing the double dissociation, I did find that the behavioural variant of frontotemporal dementia did present with rather specific risk-taking decision-making of its own.
But in the meantime the comparison with frontotemporal dementias is useful.
Nonetheless, this approach is being rolled out.
On 28 June 2013, the use of CANTABmobile was described as follows:
“The Guildford and Waverley Clinical Commissioning Group (CCG) is leading the use of an innovative new iPad-based memory assessment system as part of a national push to decrease dementia diagnosis waiting times and streamline the referral process. Accessed through NHS medical professionals, CANTABmobile enables GPs to test a patient’s episodic memory through an easy to use and administer 10-minute cognitive assessment.”
The CANTAB paired associates learning test is pictured under the heading “intuitive touchscreen interface”. if you go to “download information” on this page.
It was covered in the national media here: for example Victoria MacDonald’s report (this page provides a criticism of another report by Victoria MacDonald this time over Prof Brian Jarman’s proposed HSMR data by NHS Consultant, Dr Jacky Davis).
So what does this task test?
In understanding how the task works in reality, I found a paper where Prof Ed Bullmore and colleagues put individuals with Alzheimer’s disease and control subjects performing the task into a scanner really helpful. Bullmore and colleagues frontloaded their discussion with the following comment:
“Independent of the level of difficulty, the majority of subjects in both groups activated a network of brain regions, including the anterior cingulate, lateral, and medial occipitoparietal and frontal cortices, during successful encoding and retrieval.”
This is interesting as it doesn’t point to the usual suspects of the narrative, i.e. the entorhinal cortex and other parts of temporal lobe. Even Andrew Blackwell had described how the damage to the entorhinal cortex might possibly account dor deficits on the paired associates task:
“The transentorhinal region is a complex transitional area located between the entorhinal region proper and the adjacent temporal isocortex. It has been suggested that damage to this site in early [Alzheimer’s disease] disrupts reciprocal connections with the hippocampal formation and that this disruption underlies deficits in episodic memory.”
But on reflection is this wholly a surprise? Ed Bullmore and colleagues from their results, also from Cambridge, discuss that the lateral parietal activations reported during episodic memory tasks are thought to reflect recognition processes and retrieval processing of spatial information. Medial parietal activity has been proposed to underlie imagery and retrieval success.
I don’t feel it’s altogether surprising given what is known about the build-up of pathology in Alzheimer’s disease, either. The authors of one study looking at this report that:
“[18F]FDDNP-PET signal was significantly higher across widespread cortical regions in subjects with poorer neuropsychological test performances. Strong correlations were seen in the entorhinal, orbitofrontal, and lateral temporal cortices, temporoparietal and perisylvian language areas, parietal association cortices, and much of the dorsolateral prefrontal cortex.”
But the Sahakian lab elsewhere did find something was up with the parts in “the hippocampus and associated structures”, i.e. the structures in the temporal lobe, in this task.
But that study was only comparing MCI with normal controls. It did not include patients with Alzheimer’s disease. This is relevant, if you happen to believe that MCI ‘predates’ Alzheimer’s disease, as the authors of that study clearly do:
“Later in the course of the transition from MCI to clinical Alzheimer’s disease, functioning of the MTL deteriorates further to an extent that such compensatory activity is no longer possible. The hyperactivity in early MCI might then represent a possible predictor or biomarker of the progression to Alzheimer’s disease.”
But in the real world this is far from clear.
However, the evidence of progression of MCI (mild cognitive impairment) to DAT is currently weak. It might be attractive to think that MCI is a preclinical form of dementia of Alzheimer Type, but unfortunately the evidence is not there to back this claim up at present: most people with MCI will not progress to dementia even after ten years of follow-up (Mitchell and Shiri-Feshki, 2009). Drug companies have been trying hard to push the identification of “biomarkers”, possibly subtle psychological ‘deficits’, scan results or changes in substances in the fluid surrounding the brain (or cerebrospinal fluid). It is no accident that psychological testing and biomarkers were heavily promoted in David Cameron’s G8 dementia speech in Lancaster House at the end of last year.
In summary, I don’t think it can be taken as red that entorhinal cortex problems are causing the observed deficits in the CANTABmobile paired associates learning task.
Conclusion
Overall, my personal view is that the deficits on the CANTAB paired associates learning task are real in early Alzheimer’s disease, but possibly not for the reasons felt by some in their groups. Above all, I don’t care as such, as long as greater numbers of people benefit from a correct diagnosis of Alzhemer’s disease, but I do feel that the logic in their reasoning has gone a bit awry.
My academic viewpoint is utterly irrelevant actually, as above all I wish the whole of the medical profession well in their “war against dementia”.
I’d be the first to admit I’ve got it wrong. I am simply raising the issues in a constructive way that I hope is beneficial for the public interest.
But Dr Mitul Mehta, Reader in Neuroimaging at the IoP, does have his concerns.